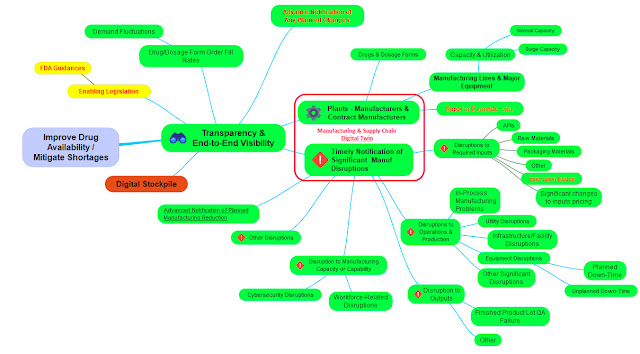
Data from the various manufacturers’ digital twins would regularly (e.g. daily) ‘feed’ a government-controlled ‘𝐩𝐡𝐚𝐫𝐦𝐚 𝐢𝐧𝐝𝐮𝐬𝐭𝐫𝐲 𝐦𝐚𝐧𝐮𝐟𝐚𝐜𝐭𝐮𝐫𝐢𝐧𝐠 𝐚𝐧𝐝 𝐬𝐮𝐩𝐩𝐥𝐲 𝐜𝐡𝐚𝐢𝐧 𝐝𝐢𝐠𝐢𝐭𝐚𝐥 𝐭𝐰𝐢𝐧.’
This will require enabling legislation e.g. to overcome manufacturer claims of proprietary information, trade secrets, etc. that they historically have used to not provide information. A wide variety of “carrots” and "sticks" will need to be used to gain industry cooperation, for example the government underwriting the costs of development, deployment, and implementation, with the benefits of having such a system accruing to the manufacturers.
Once the FDA’s QMMP is deployed to manufacturers its data can then also be folded into the digital twin. With the resulting complete transparency and end-to-end visibility across the entire industry now available to the government (manufacturing information, quality information, supply chain information, etc.), the use of AI, ML, and other such tools should support the ability to forecast and anticipate problems, issues, disruptions, etc. so that the appropriate efforts at prevention, mitigation, etc. can be carried out successfully.
6-part article of which the above is a sub-part
6-part article of which the above is a sub-part


No comments:
Post a Comment